Authorisation of pilot trials under Art. 8a NarcA
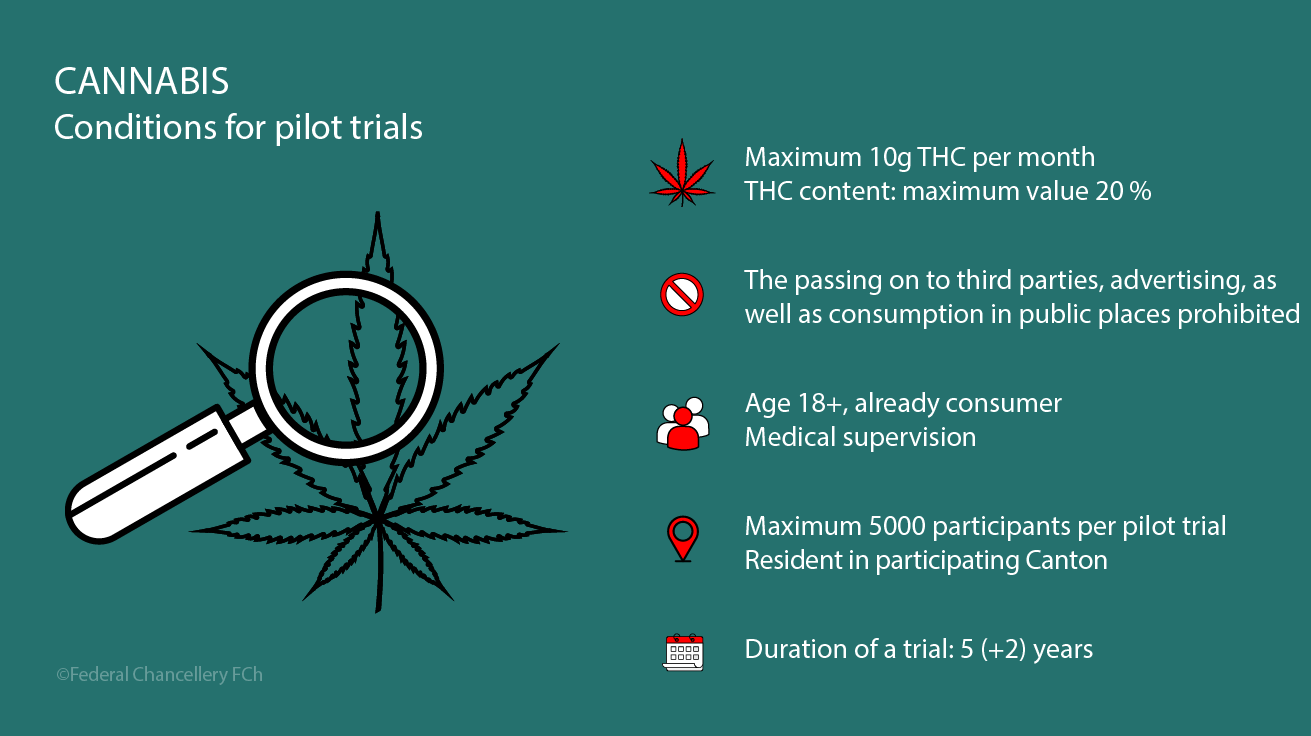
The FOPH can grant authorisations for scientific pilot trials involving the controlled dispensing of non-medicinal cannabis that are limited in time, place and scope, provided new insights into cannabis use can be gained.
The FOPH authorises pilot trials under the Narcotics Act (NarcA) provided the submitted application is complete and the requirements for conducting the trial, as stipulated in Art. 8a NarcA and the Ordinance on Pilot Trials Conducted Under the Narcotics Act (NarcPT), (in German, French and Italian) are met.
Pilot trials are scientific studies designed to deliver insights on the impact of measures, usefulness of instruments and effectiveness of approaches regarding the use of non-medicinal cannabis.
As stipulated in the Ordinance on Pilot Trials under the Narcotics Act (BetmPV), pilot trials may only be conducted if they are intended and designed to gain scientific knowledge in the areas specified under BetmPV Article 2. In accordance with BetmPV Article 23, Paragraph 2, the FOPH will reject any requests for pilot trials which are unlikely to produce any new or further findings. The FOPH enjoys a certain discretionary scope when making the corresponding assessments.
New pilot trial requests will only be considered if it can be convincingly argued that the issues they address have not been covered by existing projects. It is the responsibility of the requesting party to adequately assess this in advance.
From a regulatory perspective, further pilot trials which are expected to produce new or further findings are of primary interest in the areas of:
- Pricing actions (incentive taxes), e.g. what incentive taxes might lower consumption without demand migrating to black-market sources, and what taxes might encourage a shift to lower-risk products and forms of consumption?
- Online sales channels, e.g. how can consumers be effectively sensitised, advised and protected from health harm in this sales environment, and what restrictions would this require?
- Road safety (as part of supplementary studies): e.g. can more reliable indicators be found than the level of THC in blood to determine fitness to drive following cannabis consumption, and what other actions could be taken to enhance road safety?
- Distribution models in rural areas, e.g. what sales models could be cost-effectively adopted in rural areas, and how acceptable are these likely to be among consumers and the broader population?
The FOPH reserves the right to reject any requests for pilot trials which are unlikely to provide significant new findings.
You can find more information on pilot trials with cannabis on this page:
The authorisation procedure includes a comprehensive examination of the legal requirements, coordination of the decisions with the ethics committees and, last but not least, consultation with the cantons and communes concerned. Experience shows that several rounds of clarification with the applicants are necessary. Due to the complexity of the authorisation procedure, a processing period of at least 3 months is to be expected.
Further information
Further information
Pilot trials with cannabis
On 15 May 2021 an amendment to NarcA has come into force allowing pilot trials involving the dispensing of cannabis for non-medicinal (“recreational”) purposes. These studies are intended to create the basis for the future legal regulation of cannabis.
Exceptional licences related to pilot trials
After consulting with the relevant cantons and communes, the FOPH can authorise scientific pilot trials with narcotics containing an effective concentration of cannabinoids.
Federal Office of Public Health FOPH
Schwarzenburgstrasse 157
Switzerland - 3003 Bern